VEMLIDY is a preferred first-line therapy for adults with chronic hepatitis B, as recommended by 5 treatment guidelines/algorithms1-5
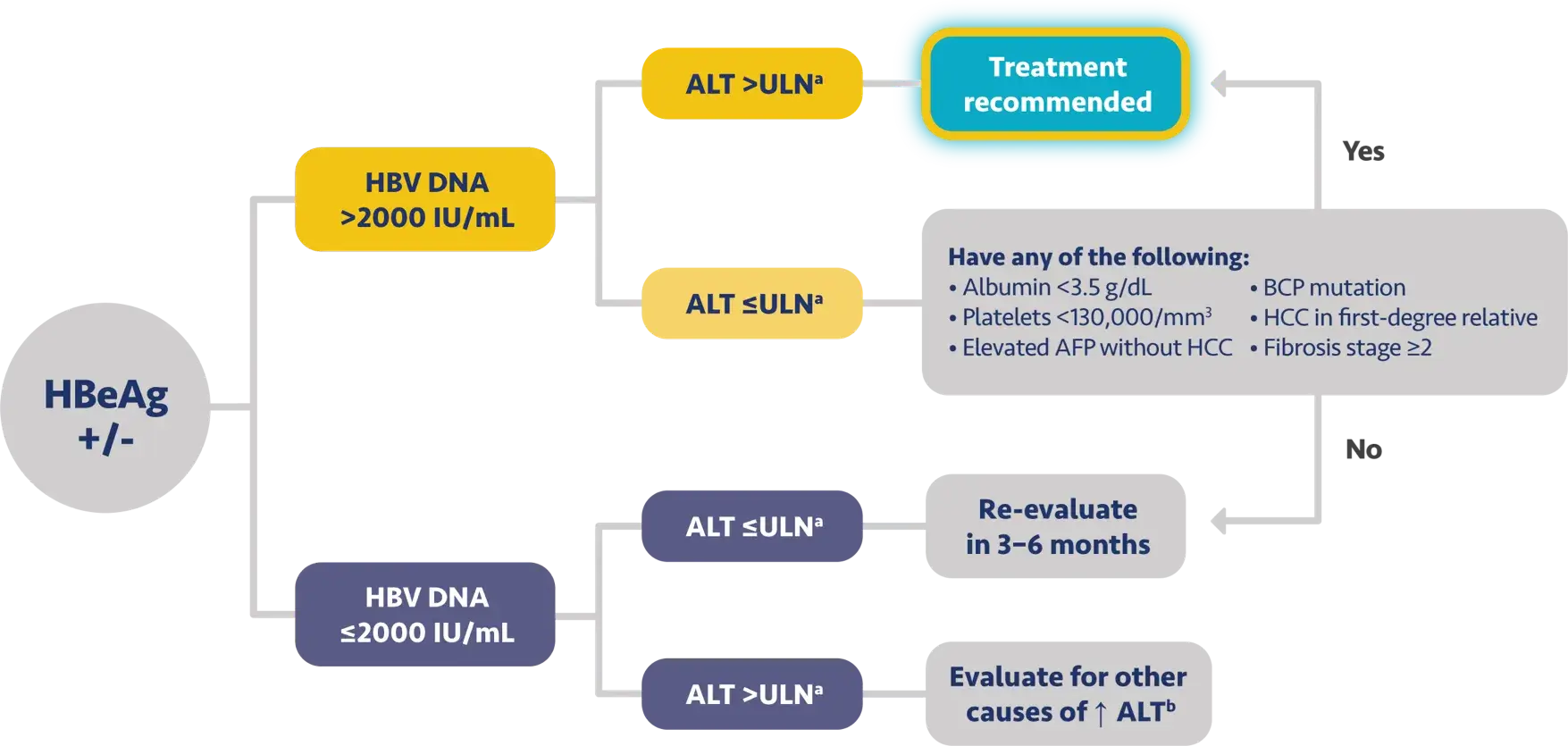
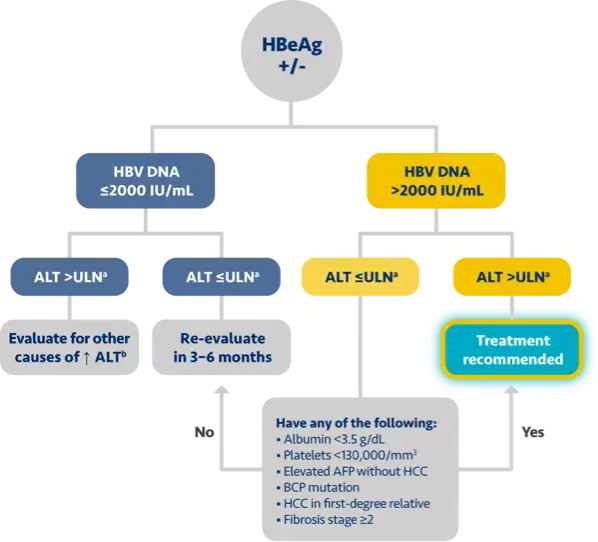
Key guideline-based criteria for treatment of chronic hepatitis B
SABA 20221
USTA 20212
Population
≥30 years olda
HBeAg+HBeAg-
(IU/mL)
HBV DNA
>2000
≥2000≥2000
ALT (U/L)
Regardless of ALT level
>ULN* or if fibrosis present/other risk factorsb>ULN* or if fibrosis present
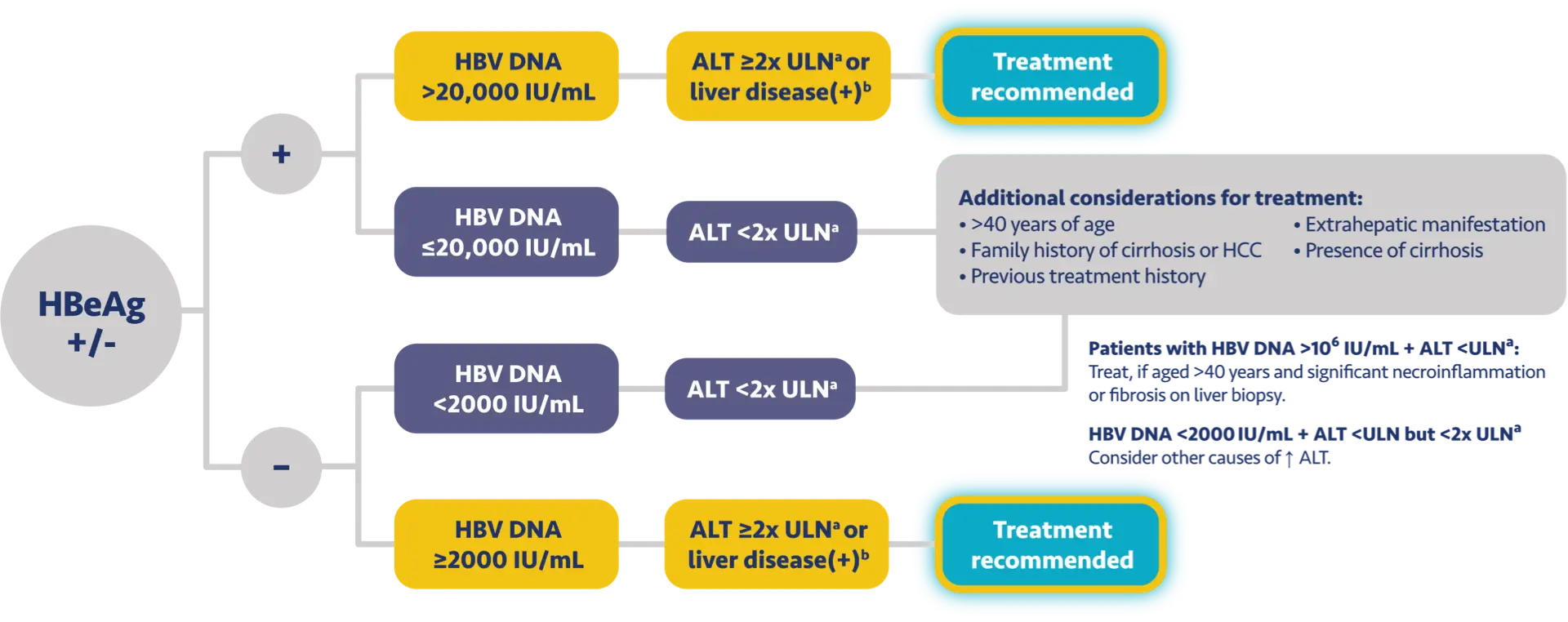
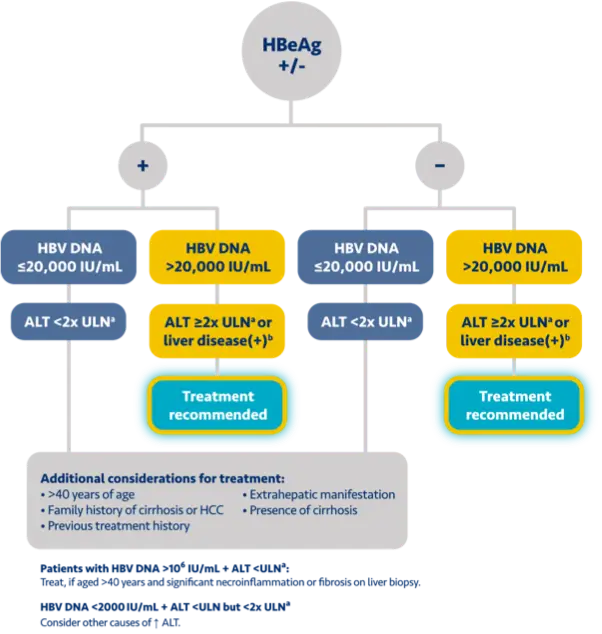
AATA 20183
AASLD 20184
Population
HBeAg+/-
HBeAg+HBeAg-
(IU/mL)
HBV DNA
>2000
>20,000>2000
ALT (U/L)
>ULN* or significant liver diseaseb/other risk factorsc,d
≥2x ULN* or significant liver diseaseb,e
Population
HBV DNA (IU/mL)
ALT (U/L)
SABA 20221
≥30 years olda
>2000
Regardless of ALT level
USTA 20212
HBeAg+HBeAg-
≥2000
>ULN* or if fibrosis present/other risk factorsb>ULN* or if fibrosis present
AATA 20183
HBeAg+/-
>2000
>ULN* or significant liver diseaseb/other risk factorsc,d
AASLD 20184
HBeAg+HBeAg-
>20,000>2000
≥2x ULN* or significant liver diseaseb,e
The 2022 SABA guideline was funded by Gilead Sciences, Inc., and developed independently by the SABA panel.
The development of the 2018 AATA was supported, in part, by an independent grant from Gilead Sciences, Inc.
Chronic hepatitis B patients with compensated cirrhosis (HBeAg+/-) and detectable HBV DNA should be treated, regardless of ALT levels1-5
ALT=alanine aminotransferase; HBeAg=hepatitis B envelope antigen; ULN=upper limit of normal.
*ULN criteria for men and women, respectively: SABA 2022: 30 U/L and 19 U/L; USTA 2021: 30 U/L and 19 U/L; AATA 2018: local laboratory range; AASLD 2018: 35 U/L and 25 U/L.1-4
aSABA recommends treatment for patients <30 years old if they have HBV DNA >2000 IU/mL and ALT >ULN.1
bNoninvasive testing showing significant fibrosis (≥F2) or liver biopsy showing moderate/severe inflammation (A2 or A3) and/or significant fibrosis (≥F2).2-4
cConsider treatment based on risk factors for developing HCC as well as patient’s age, lifestyle, and desire to undergo treatment.3
dAlbumin <3.5 g/dL, platelet count <130,000/mm3, presence of basal core promoter mutation, HCC in first-degree relative, or elevated AFP in the absence of HCC.3
eTreatment can be considered for those >40 years of age, with a family history of cirrhosis or HCC, previous treatment history, or extrahepatic manifestations (presence of extrahepatic manifestation is an indication for treatment, independent of liver disease severity).4
Watch hep B experts discuss the Simplified Approach Hepatitis B Algorithm
Guideline-based recommendations for chronic HBV management in specific patient populations


This chart does not include the complete prescribing and dosing considerations for using these medications. Please refer to the full Prescribing Information for each medication. Comparison of drugs does not imply comparable clinical effectiveness, safety, or tolerability. Individual prescribing decisions should be made at the discretion of the provider.
VEMLIDY has not been tested for use during pregnancy. Both AASLD and EASL guidelines recommend TDF for use during pregnancy.4,5
fVEMLIDY is not recommended in patients with end stage renal disease (ESRD; estimated creatinine clearance [eCrCl] <15 mL/min) who are not receiving chronic hemodialysis; in patients on chronic hemodialysis, on hemodialysis days, administer VEMLIDY after completion of hemodialysis treatment.6
gVEMLIDY is not indicated for patients with decompensated (Child-Pugh B or C) hepatic impairment and has not been tested in this population.6
hPostmarketing cases of renal impairment, including acute renal failure, proximal renal tubulopathy (PRT), and Fanconi syndrome have been reported with TAF-containing products. Patients with impaired renal function and/or taking nephrotoxic agents (including NSAIDs) are at increased risk of renal-related adverse reactions. Discontinue VEMLIDY in patients who develop clinically significant decreases in renal function or evidence of Fanconi syndrome. Monitor renal function in all patients. See Dosage and Administration.6
iVEMLIDY and entecavir are preferred in chronic hepatitis B patients with deteriorating renal function or low eGFR and/or osteopenia/osteoporosis, particularly in older age. Per EASL, VEMLIDY is preferred to entecavir in patients with previous exposure to lamivudine.5
jGeneric TDF and generic entecavir are therapeutically equivalent to the respective brand name drug. These generic products are designated Therapeutic Equivalence Code AB, which the FDA considers therapeutically equivalent to other pharmaceutically equivalent products. Please refer to the appropriate manufacturers of generic TDF and generic entecavir for the full Prescribing Information.
kTDF 300 mg does not require dosing adjustment in patients with eCrCl ≥50 mL/min. For patients with eCrCl 30-49 mL/min, 10-29 mL/min, and ESRD on chronic hemodialysis, the dosing is every 48 hours, every 72-96 hours, and every 7 days or after a total of approximately 12 hours of dialysis, respectively.7
lEntecavir 0.5 mg does not require dosing adjustment in patients with eCrCl ≥50 mL/min. For patients with eCrCl 30-49 mL/min, 10-29 mL/min, and ESRD on chronic hemodialysis, the dosing is 0.25 mg once daily or 0.5 mg/48 hours, 0.15 mg once daily or 0.5 mg/72 hours, and 0.05 mg once daily or 0.5 mg/7 days, respectively.8 Please refer to the Prescribing Information for additional dosing information for entecavir 1 mg in patients with lamivudine-refractory or decompensated liver disease.8
IMPORTANT SAFETY INFORMATION
BOXED WARNING: POSTTREATMENT SEVERE ACUTE EXACERBATION OF HEPATITIS B
- Discontinuation of anti-hepatitis B therapy, including VEMLIDY, may result in severe acute exacerbations of hepatitis B. Hepatic function should be monitored closely with both clinical and laboratory follow-up for at least several months in patients who discontinue anti-hepatitis B therapy, including VEMLIDY. If appropriate, resumption of anti-hepatitis B therapy may be warranted.
Warnings and Precautions
- Risk of Development of HIV-1 Resistance in HBV/HIV-1 Coinfected Patients: Due to this risk, VEMLIDY alone should not be used for the treatment of HIV-1 infection. Safety and efficacy of VEMLIDY have not been established in HBV/HIV-1 coinfected patients. HIV antibody testing should be offered to all HBV-infected patients before initiating therapy with VEMLIDY, and, if positive, an appropriate antiretroviral combination regimen that is recommended for HBV/HIV-1 coinfected patients should be used.
- New Onset or Worsening Renal Impairment: Postmarketing cases of renal impairment, including acute renal failure, proximal renal tubulopathy (PRT), and Fanconi syndrome have been reported with TAF-containing products. Patients with impaired renal function and/or taking nephrotoxic agents (including NSAIDs) are at increased risk of renal-related adverse reactions. Discontinue VEMLIDY in patients who develop clinically significant decreases in renal function or evidence of Fanconi syndrome. Monitor renal function in all patients – See Dosage and Administration.
- Lactic Acidosis and Severe Hepatomegaly with Steatosis: Fatal cases have been reported with the use of nucleoside analogs, including tenofovir disoproxil fumarate (TDF). Discontinue VEMLIDY if clinical or laboratory findings suggestive of lactic acidosis or pronounced hepatotoxicity develop, including hepatomegaly and steatosis in the absence of marked transaminase elevations.
Adverse Reactions
Most common adverse reactions (incidence ≥5%; all grades) in clinical studies through week 144 were headache, upper respiratory tract infection, abdominal pain, cough, back pain, arthralgia, fatigue, nausea, diarrhea, dyspepsia, and pyrexia.
Drug Interactions
- Coadministration of VEMLIDY with drugs that reduce renal function or compete for active tubular secretion may increase concentrations of tenofovir and the risk of adverse reactions.
- Coadministration of VEMLIDY is not recommended with the following: oxcarbazepine, phenobarbital, phenytoin, rifabutin, rifampin, rifapentine, or St. John’s wort. Such coadministration is expected to decrease the concentration of tenofovir alafenamide, reducing the therapeutic effect of VEMLIDY. Drugs that strongly affect P-glycoprotein (P-gp) and breast cancer resistance protein (BCRP) activity may lead to changes in VEMLIDY absorption.
Consult the full prescribing information for VEMLIDY for more information on potentially significant drug interactions, including clinical comments.
Dosage and Administration
- Testing Prior to Initiation: HIV infection.
- Prior to or When Initiating, and During Treatment: On a clinically appropriate schedule, assess serum creatinine, estimated creatinine clearance, urine glucose, and urine protein in all patients. In patients with chronic kidney disease, also assess serum phosphorus.
- Dosage in Adults: 1 tablet taken once daily with food.
- Renal Impairment: Not recommended in patients with end stage renal disease (ESRD; eCrCl <15 mL/min) who are not receiving chronic hemodialysis; in patients on chronic hemodialysis, on hemodialysis days, administer VEMLIDY after completion of hemodialysis treatment.
- Hepatic Impairment: Not recommended in patients with decompensated (Child-Pugh B or C) hepatic impairment.
Pregnancy and Lactation
- Pregnancy: A pregnancy registry has been established for VEMLIDY. Available clinical trial data show no significant difference in the overall risk of birth defects for VEMLIDY compared with the background rate of major birth defects in the U.S. reference population.
- Lactation: TAF and tenofovir can pass into breast milk. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for VEMLIDY and any potential adverse effects on the breastfed infant from VEMLIDY or from the underlying maternal condition.
INDICATION
VEMLIDY is indicated for the treatment of chronic hepatitis B virus (HBV) infection in adults with compensated liver disease.
Please see full Prescribing Information for VEMLIDY, including BOXED WARNING.